[ad_1]
• Analysis Spotlight
Fragile X syndrome (FXS) is a genetic dysfunction brought on by the gene FMR1. It’s the most typical type of inherited mental incapacity and infrequently co-occurs with different situations like autism and epilepsy.
New analysis has unveiled main insights into genetic mechanisms underlying FXS and associated problems. The research discovered that the problems contain intensive silencing of many genes that play key roles in constructing tissues and making the mind work correctly.
The analysis was funded by the Nationwide Institute of Psychological Well being, the Nationwide Institute of Neurological Problems and Stroke, the Eunice Kennedy Shriver Nationwide Institute of Baby Well being and Human Growth, and the NIH Frequent Fund’s 4D Nucleome Program.
How do adjustments to the FMR1 gene result in FXS?
The prevailing concept is that FXS includes adjustments to FMR1, a gene positioned on the X chromosome (considered one of two intercourse chromosomes in people). The primary a part of the FMR1 gene is made up of repeats of a selected DNA sequence known as a CGG. The CGG sequence can increase uncontrollably, resulting in an extra variety of repeats. A traditional-length FMR1 sequence has lower than 40 CGG repeats. In distinction, a mutation-length FMR1 sequence has over 200 CGG repeats.
When the FMR1 CGG reaches this mutation size, it causes bodily adjustments to the gene that silence its expression. Because of this, FMR1 produces solely slightly or none of its protein, which is required for wholesome mind operate. That is when FXS emerges. Individuals with FXS missing the FMR1 protein can expertise important impacts on their improvement , together with mental and studying disabilities; speech and language difficulties; and social and behavioral issues, resembling hyperactivity and anxiousness.
Nevertheless, adjustments in FMR1 alone don’t account for the complete vary of FXS signs, as evidenced by mouse fashions wherein the Fmr1 gene is “knocked out,” or genetically turned off. Earlier analysis has additionally implicated a broader set of genetic mechanisms and gene areas in FMR1 silencing.
What did researchers do within the present research?
Jennifer Phillips-Cremins, Ph.D. , and the research’s first authors, Thomas Malachowski, M.S., Keerthivasan Chandradoss, Ph.D., Ravi Boya, Ph.D., and Linda Zhou, M.D., Ph.D., on the College of Pennsylvania led researchers in wanting past the usual mannequin of FXS. They examined whether or not human FXS tissues present adjustments within the genome (the entire set of genetic materials [DNA]) or the epigenome (chemical modifications that affect gene expression with out altering the DNA sequence) and whether or not these adjustments are particular to FMR1 or have an effect on different genes as nicely.
The researchers mixed a number of high-tech analytic strategies, together with molecular mapping, DNA imaging, epigenetic sequencing, and genetic engineering. Further computational approaches allowed them to combine and discover patterns within the giant datasets they used.
The researchers examined genetic, epigenetic, and imaging information in a number of human cell traces from individuals with FXS and other people with out the dysfunction. They appeared genome-wide for adjustments occurring on each the X chromosome and non-sex chromosomes (generally known as autosomes ). As well as, they labored with the NIH NeuroBrainBank to gather postmortem tissue from a mind space linked to FXS—the caudate nucleus—from individuals with and with out the dysfunction.
These superior strategies enabled the researchers to take a look at the form of the DNA in the complete genome and the way it folds into complicated 3D buildings contained in the cell nucleus (generally known as the 3D genome). The folding of the genome in 3D area displays the packaging and interactions of segments of chromatin (the mix of DNA and protein that makes up the genome). Exact folding patterns within the 3D genome are important for correct gene regulation and mobile operate, and deviations from this construction are related to genetic problems like FXS in addition to many cancers.
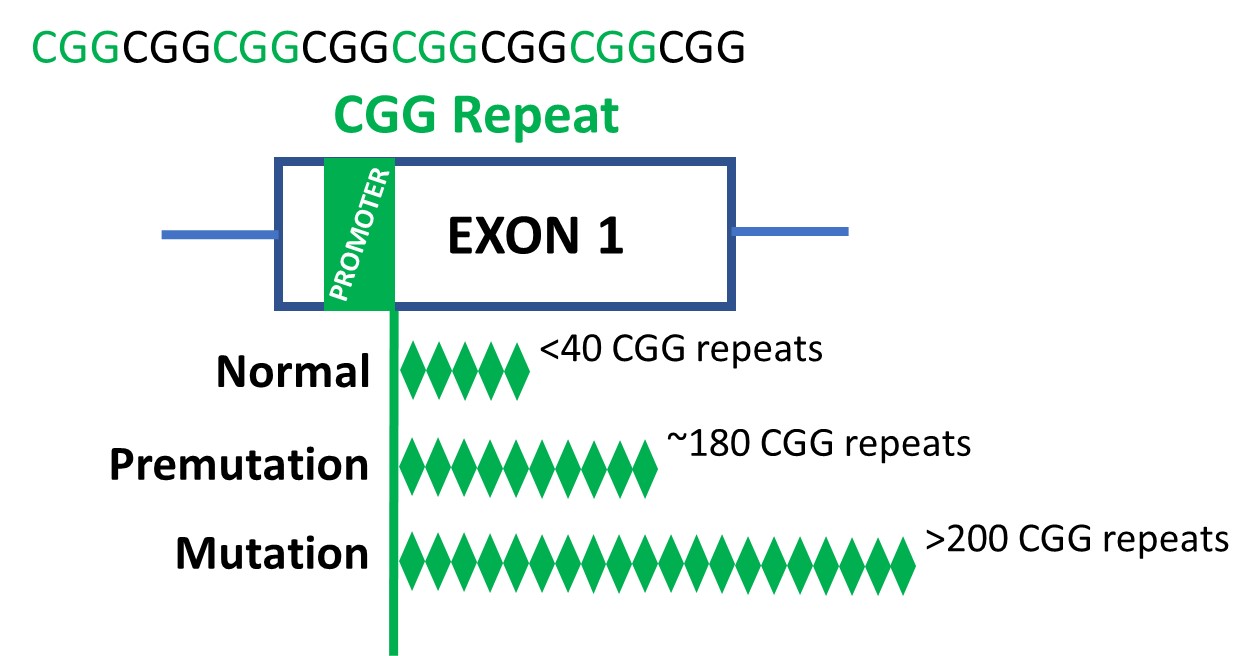
In all cell traces and tissues, the researchers in contrast the three variations of theFMR1 CGG repeat that may happen:
- Regular size (5–45 CGG repeats)
- Premutation size (61–199 CGG repeats)
- Mutation size (>200 CGG repeats; FXS)
By analyzing the 3D genome and chromatin adjustments decided by the size of the CGG repeat, the researchers may discover epigenetic adjustments and hyperlink them to gene expression silencing and genome instability that can lead to genetic problems.
What did the outcomes present?
The outcomes confirmed, in cells and tissue from individuals with FXS, beforehand identified adjustments related to the mutation-length CGG enlargement, together with epigenetic adjustments to the FMR1 gene and silencing of FMR1 gene expression. The researchers additionally unexpectedly found extra widespread adjustments to the DNA.
They discovered deposits of enormous pockets of a silencing type of chromatin, generally known as heterochromatin, that’s folded too tightly and makes the gene much less accessible. The heterochromatin pockets prolonged far past FMR1. On the X chromosome, the heterochromatin radiated outward to silence genes upstream of FMR1 which are important for neural cell capabilities resembling circuit connectivity and synaptic plasticity, which may trigger studying difficulties generally skilled by individuals with FXS. On autosomal chromosomes, the heterochromatin silenced a number of genes associated to pores and skin, tendon, and ligament integrity, that are clinically affected tissues in individuals with FXS.
The researchers additionally discovered extreme misfolding of DNA and potential websites of breakage alongside the DNA sequence or slight expansions of different repetitive sequences inside the heterochromatin. All these larger order genome folding patterns inside cells are important for correct gene operate, so the recognized adjustments in genome construction are possible related to FXS past the native silencing of FMR1.
The Cremins Laboratory coined the time period beacons of repeat enlargement anchored by contacting heterochromatin, or BREACHes, to discuss with the community of silenced gene areas they found, marked by extreme chromatin misfolding and genome instability. The researchers suggest that the organic significance of BREACHes is silencing genes concerned in processes important for mind operate, together with ones controlling synaptic plasticity that allows the mind to vary in response to studying and different dynamic conditions. Silencing of those genes could assist clarify lots of the developmental variations seen in individuals with FXS.
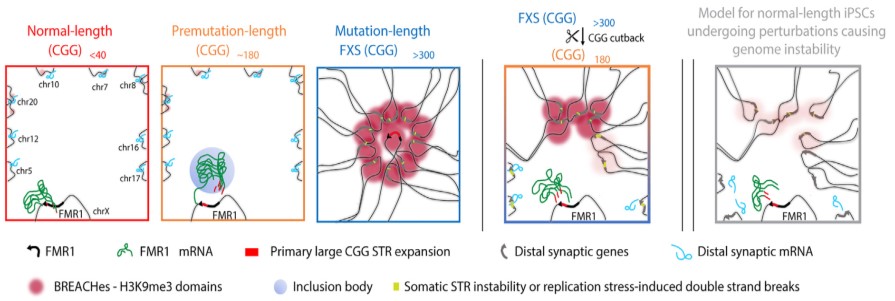
Having established broad structural and purposeful adjustments brought on by the mutation-length FMR1 CGG, the researchers checked out what occurred once they reduce the repeat to premutation size. They discovered that shortening the CGG size led to the elimination of heterochromatin on lots of the BREACHes. This discovering signifies that the size of the CGG repeat is a vital contributor to at the least among the extreme and widespread 3D genome misfolding and gene silencing seen in FXS and associated problems. It additionally means that reverse-engineering the mutation-length CGG repeat may probably forestall the emergence of genome-wide defects seen in frequent genetic problems in people.
What do the outcomes imply?
Collectively, the outcomes reveal important adjustments within the genome construction associated to the mutation-length FMR1 CGG sequence. These adjustments, related to issues in how the DNA folds and instability within the genome, precipitated genes with necessary roles in mind operate to turn out to be silent or inactive, serving to clarify lots of the signs seen in individuals with FXS.
The silenced areas spanned a community of widespread gene areas that the researchers known as BREACHes. BREACHes had been noticed in a number of cell sorts and postmortem mind tissue from individuals with FXS and on each the X chromosome and a number of autosomal chromosomes. BREACHes additionally included a number of genes not beforehand linked to FXS, providing new targets for future investigation.
In response to the researchers, the invention of BREACHes fills a significant lacking piece in understanding FXS. The novel discovering helps clarify the scientific presentation and frequent signs of individuals with FXS, which couldn’t beforehand be defined by lack of the FMR1 protein alone. As a result of genome misfolding occurred not solely in FXS cell traces but additionally in cells with instability generally, BREACHes is likely to be significant to the rising checklist of problems marked by unstable repeat expansions.
This research enhances understanding of the genetic and epigenetic mechanisms contributing to FXS illness pathology. The outcomes could, in time, have broad relevance for understanding, diagnosing, and even treating the numerous problems marked by unstable repeat expansions or genome instability, together with FXS and most cancers.
Reference
Malachowski, T., Chandradoss, Ok. R., Boya, R., Zhou, L., Prepare dinner, A. L., Su, C., Pham, Ok., Haws, S. A., Kim, J. H., Ryu, H.-S., Ge, C., Luppino, J. M., Nguyen, S. C., Titus, Ok. R., Gong, W., Wallace, O., Joyce, E. F., Wu, H., Rojas, L. A., & Phillips-Cremins, J. E. (2023). Spatially coordinated heterochromatinization of lengthy synaptic genes in fragile X syndrome. Cell, 186(26), 5840–5858. https://doi.org/10.1016/j.cell.2023.11.019
Grants
MH120269 , MH129957 , DK127405 , DA052715 , HD098015 , NS129317
[ad_2]